Following the last AAV Company series post on ophthalmology another organ system is coming to the forefront of early clinical development: the central nervous system (CNS).
The playbook for AAV gene therapy to be successful in CNS disorders is not much different than other organ systems. Researchers need to overcome the same limitations: tropism for the right cell types, safety, persistence and delivery. AAV has been delivered to the CNS in many different ways. In recent years, we’ve observed success and safety with targeting the CNS via direct infusion into the brain or through the spine.
A good example of CNS delivery via the spine is the recent n-of-1 gene therapy trial for Spastic Paraplegia 50 (SPG-50), an ultra-rare orphan disease affecting less than 100 patients globally. The disease presents as a progressive neuromuscular disorder with mental disability and paralysis. A single patient was dosed in 2022 with a AAV9/AP4M1 gene therapy vector via spinal infusion and has since shown signs of improvement.
Infusion directly to the brain was explored in the late-90s and early 2000’s for much larger CNS indications like Parkinson’s, Alzheimer’s and Huntington’s. However, success was limited due to insufficient neurosurgical techniques, selecting the wrong AAV serotypes and selection of delivery site. These early mistakes and learnings informed the clinical trial for another ultra-rare disorder: aromatic L‑amino acid decarboxylase (AADC) deficiency. These patients are unable to synthesize dopamine and serotonin leading to a devastating disease phenotype. Owing to the advancements in neurosurgery and the unmet needs of this patient population, an academic led clinical trial was approved to infuse patients with an AAV2 vector directly to the brain delivering the human AADC gene. Dozens of patients have now been infused with a gene therapy (marketed by PTC Therapeutics as Upstaza), and most patients experience improvements in motor and cognitive function within 12 months with sustained neuromuscular improvements 5 years after infusion. Upstaza is being submitted for FDA approval by PTC Therapeutics, after already receiving approval in the UK and EMA likely becoming the first FDA approved CNS-targeted AAV gene therapy.
Now with growing clinical evidence of efficacy and safety in ultra-rare orphan diseases and continued research in larger indications like Parkinson’s, is the biopharma industry ready to take CNS gene therapies seriously?
That looks like where the field is going with recent breakthroughs in AAV vectors engineered for improved CNS delivery.
Voyageur Therapeutics announced back in 2021 a break-through for their CNS-directed capsid demonstrating robust delivery across the blood brain barrier in non-human primates citing a 1000-fold higher transgene expression in the brain and 100-fold higher transgene expression in the spinal cord via their TRACER platform. This led to several BD deals with Neurocine Biosciences, Novartis, and Pfizer accumulating over 500M USD in upfront and milestones payments thus far.
Recently, Sangamo Therapeutics announced their own next-generation capsid they believe is better than the Voyageur Therapeutics capsid. Future deals on this capsid is possible considering Sangamo’s relationships with both Prevail Therapeutics (owned by Eli Lilly) and Pfizer (partnered on their hemophilia A program).
The rare disease and gene therapy sector can quickly become exciting again for investors as toolkits expand opening access to other organs. The regulatory path improving can accelerate this as well, since we recently heard from the FDA on their plans to improve the pathway for accelerated approvals for gene therapies.
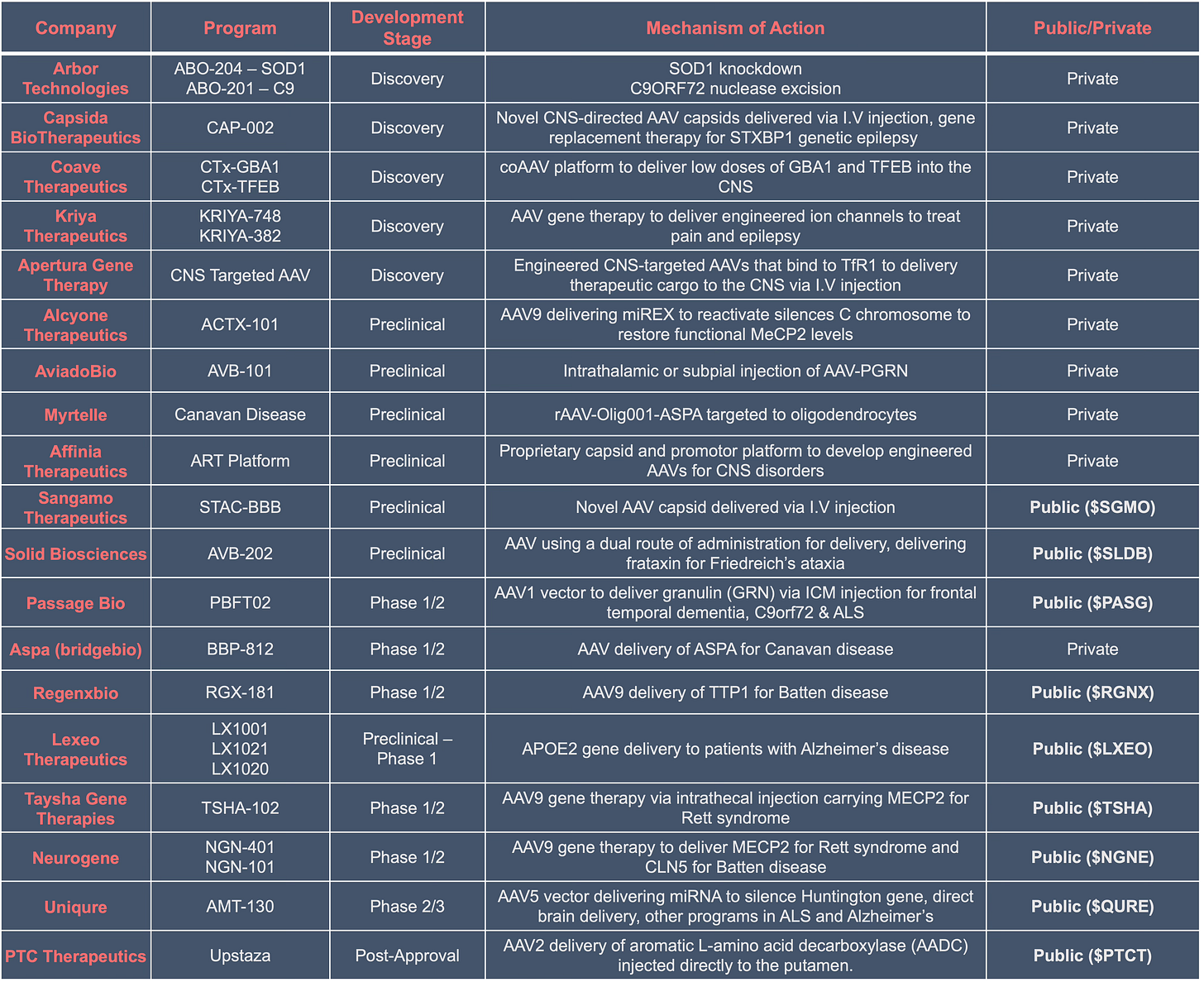
Let’s take a look at the pipeline and select players developing CNS-directed AAV therapeutics, the list is not exhaustive as there are over 100 biotechs and pharmaceutical companies in this space.