The eye has long been considered the “ideal” organ to generate proof-of-concept for corrective gene therapies. But early success with Luxturna in RPE65 mutation-associated retinal dystrophy has been slow to lead to more successful ocular gene therapies. Now 6-years later, we’re getting closer to an answer in a new indication.
Retinitis Pigmentosa (RP)
RP represents a group of inherited disorders that leads to gradual vision loss and blindness. The condition is marked by gradual degeneration of photoreceptors in the retina. As they deteriorate over time, individuals with RP experience progressive loss of peripheral vision, night blindness, and, eventually, central vision.
A Genetically Diverse, Rare Disease
One of the most challenging aspects of developing a gene therapy for RP is its genetic diversity. Over 70 genes have been identified as contributors to RP, leading companies to focus on the most common forms of RP.
Emerging Therapies
Researchers have explored gene therapy, stem cells, and retinal implants. However, there are unique challenges in delivering therapies to retinal tissue. So, it is not surprising the majority of the companies chasing this indication are using the validated AAV approach. But the success of these treatments will depend on their ability to halt or slow the degeneration of photoreceptors and potentially restore some level of vision. How confident are we that AAV vectors can do this? Is delivering a healthy copy of the gene and halting progression enough to gain FDA approval?
The Lead Players
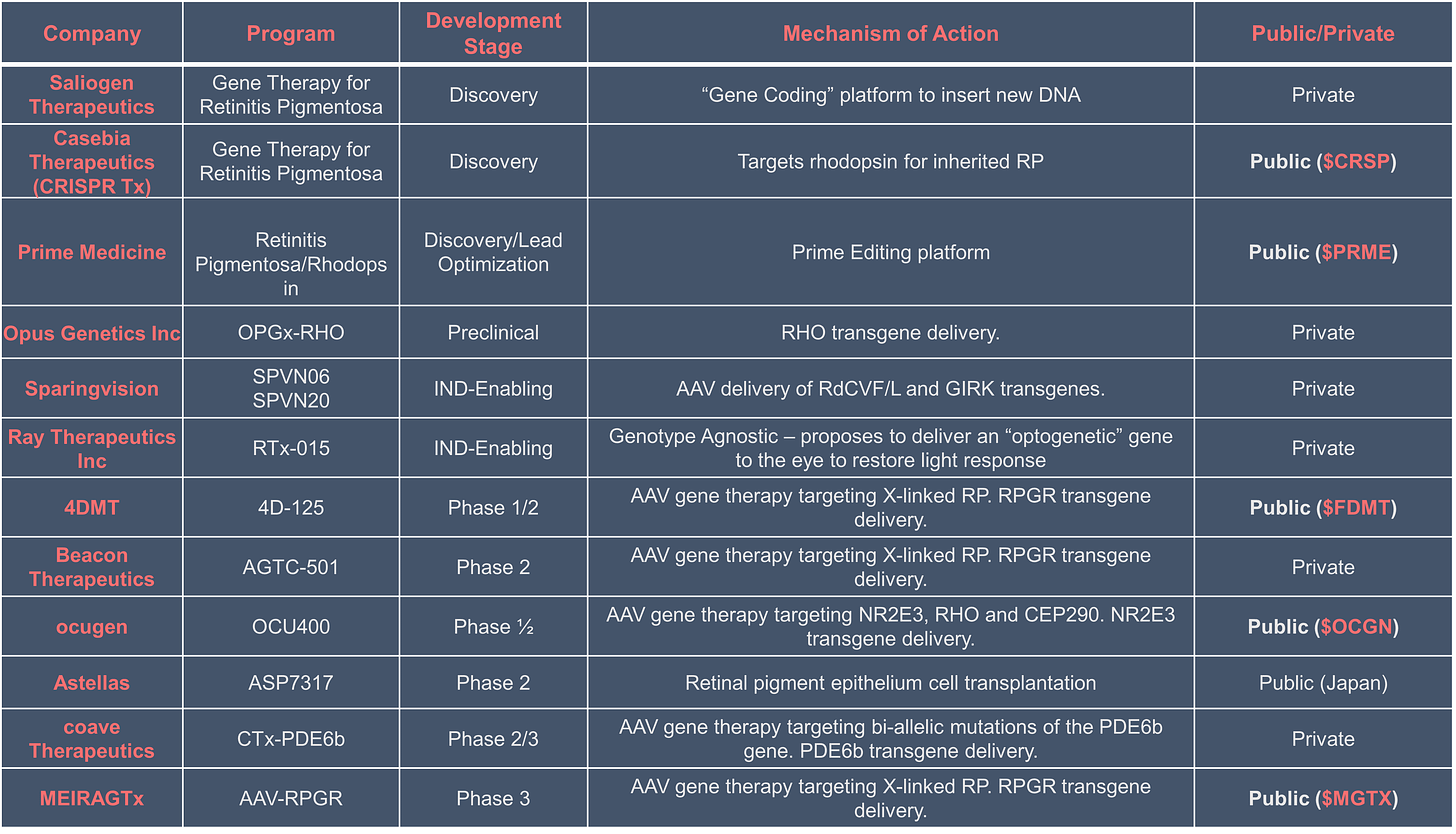
Ultimately, investors and patients are hoping to see the next Luxturna find approval in RP. With a strong unmet need in the field and the validation of AAV therapies for the eye - there is extensive competition in RP. Below are the lead companies we’ve identified:
Based on this competitive landscape, we will be following MGTX 0.00%↑ very closely over the next year.
Other public co’s in this space: OCGN 0.00%↑ , FDMT 0.00%↑ , PRME 0.00%↑ , CRSP 0.00%↑ (via Casebia Therapeutics).