Cellectar Biosciences Presents Pipeline Updates
new preclinical data following promising clinical updates
Cellectar Biosciences CLRB 0.00%↑ generated buzz recently by achieving its primary endpoint in the CLOVER WaM pivotal trial in highly refractory Waldenstrom’s macroglobulinemia for their drug Iopofosine.
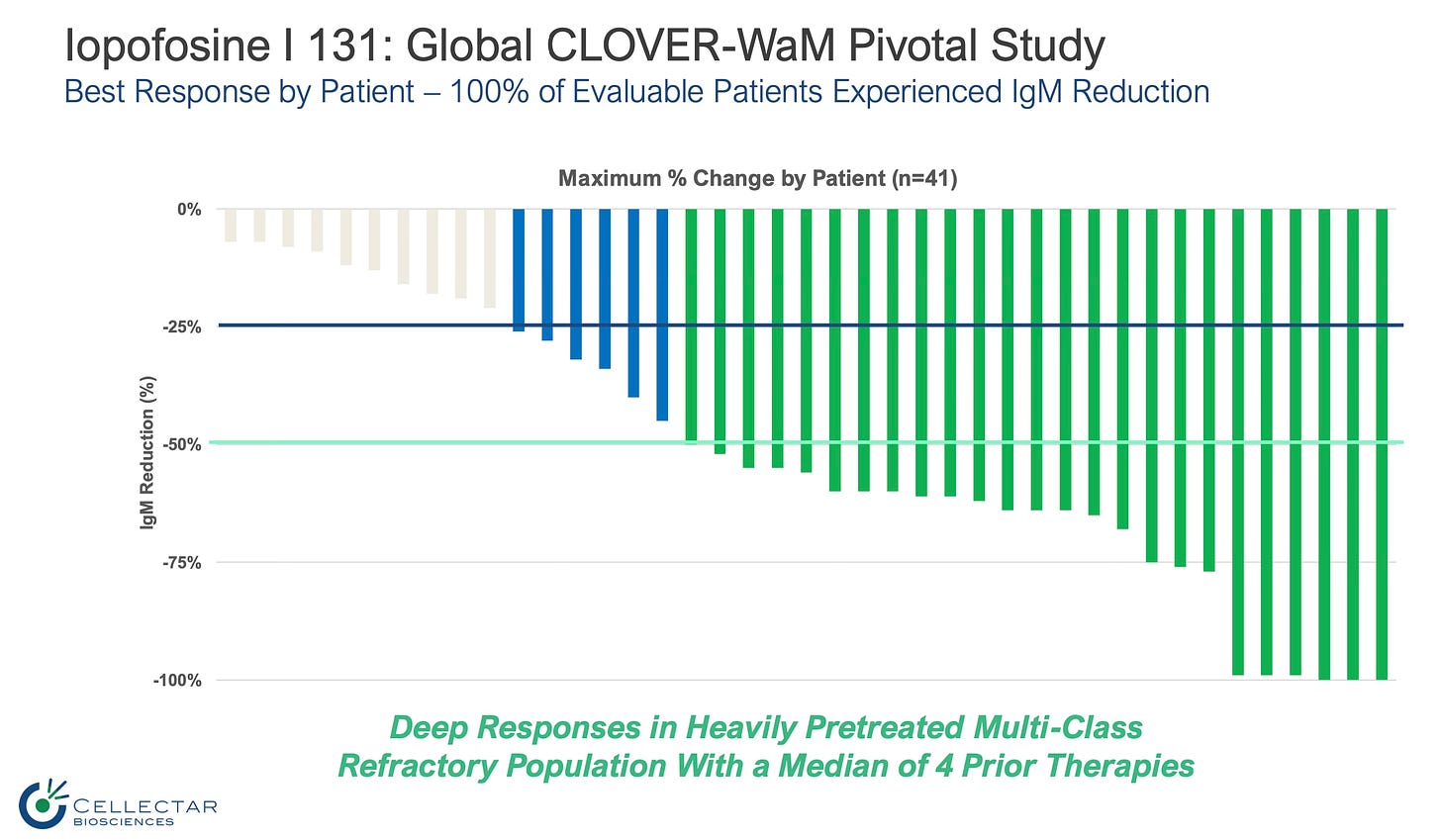
Money slide from the readout: Iopofosine, a beta-emitting phospholipid radiotherapeutic conjugate, was tested in heavily pretreated multi-class refractory population with a median of four prior therapies:
75.6% Overall Response Rate
61% Major Response Rate (95% CI, 44.5%, 75.8%)
100% Disease Control Rate exceeding protocol statistical hurdle of 20%
The data is still maturing, meaning we may see more patients respond to therapy as the trial continues.
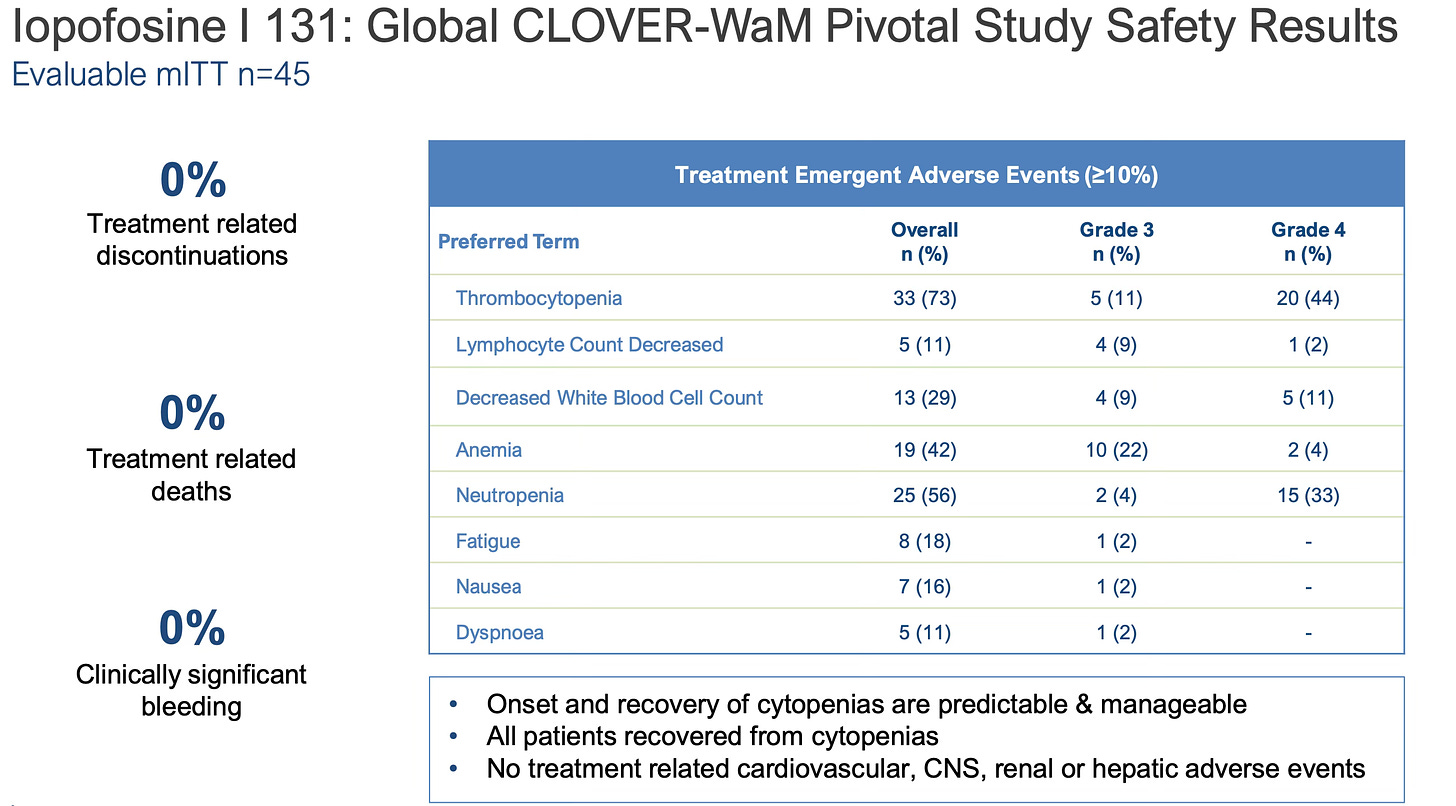
Safety was also promising with no treatment related discontinuations.
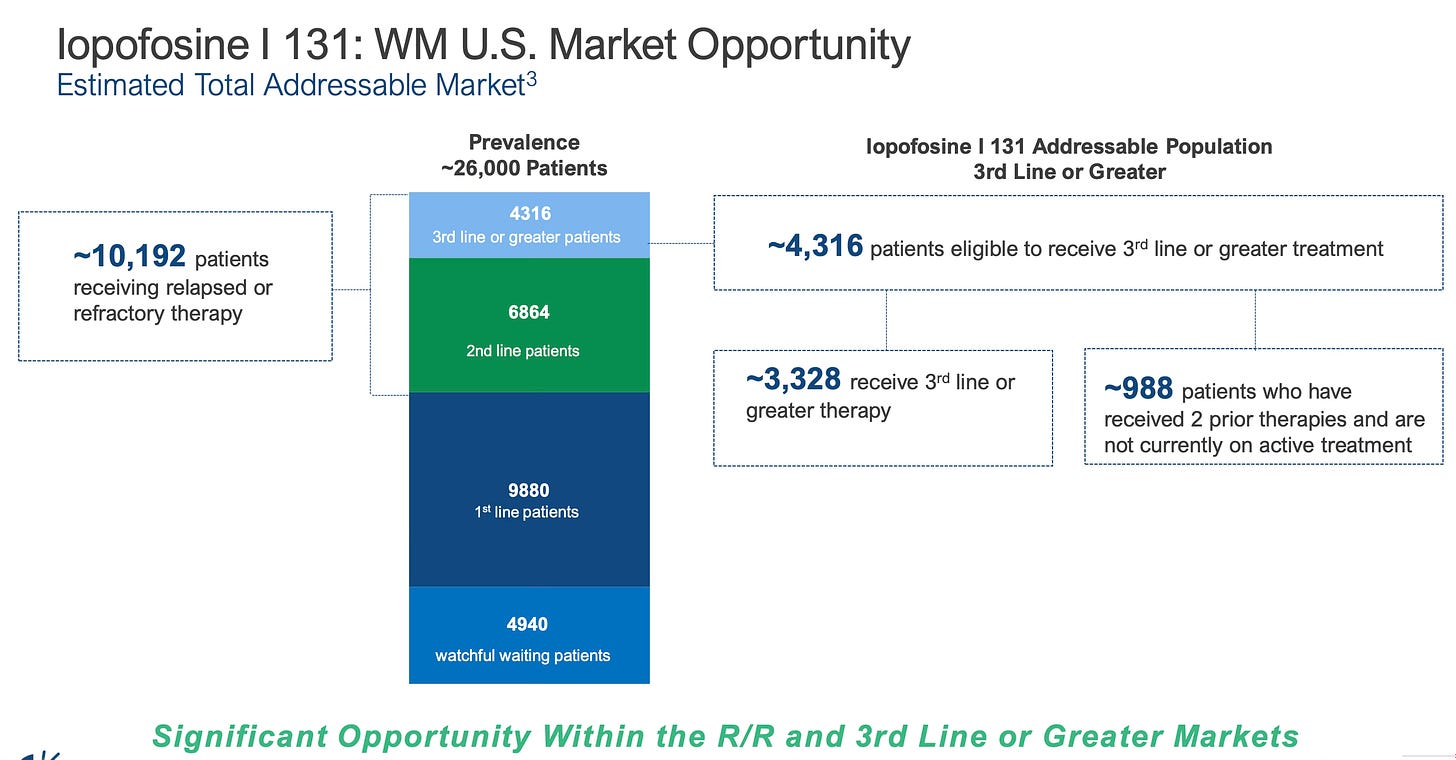
The market opportunity for Iopofosine is strong with a potential target market of 26000 patients.
New Preclinical Data
The company announced promising preclinical data for their novel alpha-emitting phospholipid radiotherapeutic conjugate, CLR 121225 (225Ac-CLR 121225) in pancreatic cancer models.
This new program will be added to the company’s clinical pipeline expanding to include targeted alpha therapies.
Competing TAT platforms, such as antibodies and peptides, possess the potential to be effective for treating cancers with low tumor volume, they are challenged to treat higher volume or bulky tumors due to insufficient penetration and the need for high quantities of the target epitope. Cellectar proposes that their compounds have biochemical properties that enable penetration of the TAT payload deep into the tumor mass and the abundance of lipid rafts on tumor cells provides near universal delivery and enhanced outcomes.
Key Data for CLR 121225:
Preclinical model: Potent anti-tumor activity in refractory pancreatic cancer mouse xenograft models
Dosage: Each dose level (100nCi, 250nCi and 500nCi) resulted in tumor volume reduction, the highest dose providing near complete eradication of the tumor.
Biodistribution: approximately 15 – 20% of the infused drug accumulated in the tumor within four hours and continued to accumulate over 72 – 96 hours.
Toxicity: no end organ toxicities demonstrating good tolerability.
People may recognize actinium-225, as the isotope was recently validated by BMS acquiring Rayze Bio for a total equity value of approximately $4.1 billion on December 26th, 2023.
While Cellectar has a long way to go to demonstrate they’re on a similar path - the story so far is looking good.