As someone who worked on rare genetic disorders during graduate school, it was gene therapy companies that first brought my attention to the world of biotech.
The biggest story around that time was Audentes Therapeutics’ X-Linked Myotubular Myopathy (XLMTM) program. Audentes was developing a gene therapy using an AAV8 vector to delivery the mutated MTM1 gene. The first results were striking - where patients would typically die early with progressive muscle failure and respiratory failure - patients achieved motor milestones such as walking and staying off ventilator support. Ultimately, Audentes was acquired by Astellas for 3B USD in 2019.
Since then the field has not progressed much despite the immense amount of work put in to develop next-generation AAV vectors that can overcome existing immunogenicity, improved targeting to specific tissues and increasing payload size.
To date the FDA has approved the following AAV gene therapies:
Luxturna (AAV2) - Leber’s Congenital amaurosis: 2017
Zolgensma (AAV9) - SMA: 2019
HEMGENIX (AAV5) - Hemophilia B: 2022
ELEVIDYS (AAVrh74) - DMD: 2023
ROCTAVIAN (AAV5) - Hemophilia A: 2023
Interestingly, ELEVIDYS is the only non-human AAV approved with no rationally designed or directed-evolution AAV capsids approved to date.
The current pipeline of AAV companies are growing in areas beyond ophthalmology and neuromuscular disorders to include metabolic, cardiac, CNS, the ear and respiratory.
As discussed in the Retinitis Pigmentosa overview the eye has been considered the ideal organ for AAV gene therapy for a few reasons: localized vector delivery to avoid systemic toxicity and off-target effects, and an immune privileged organ avoiding innate immunity. However, since the approval of Luxturna in 2017 there has not been another ocular gene therapy approved by the FDA.
Biogen took a candidate all the way to phase 3 trials but unfortunately fell short in choroideremia. The phase 3 trials published in Nature Medicine found that the subretinal injection of the AAV2-vector-based gene therapy (timrepigene emparvovec) did not meet its primary endpoint of best-corrected visual acuity (BCVA) improvement.
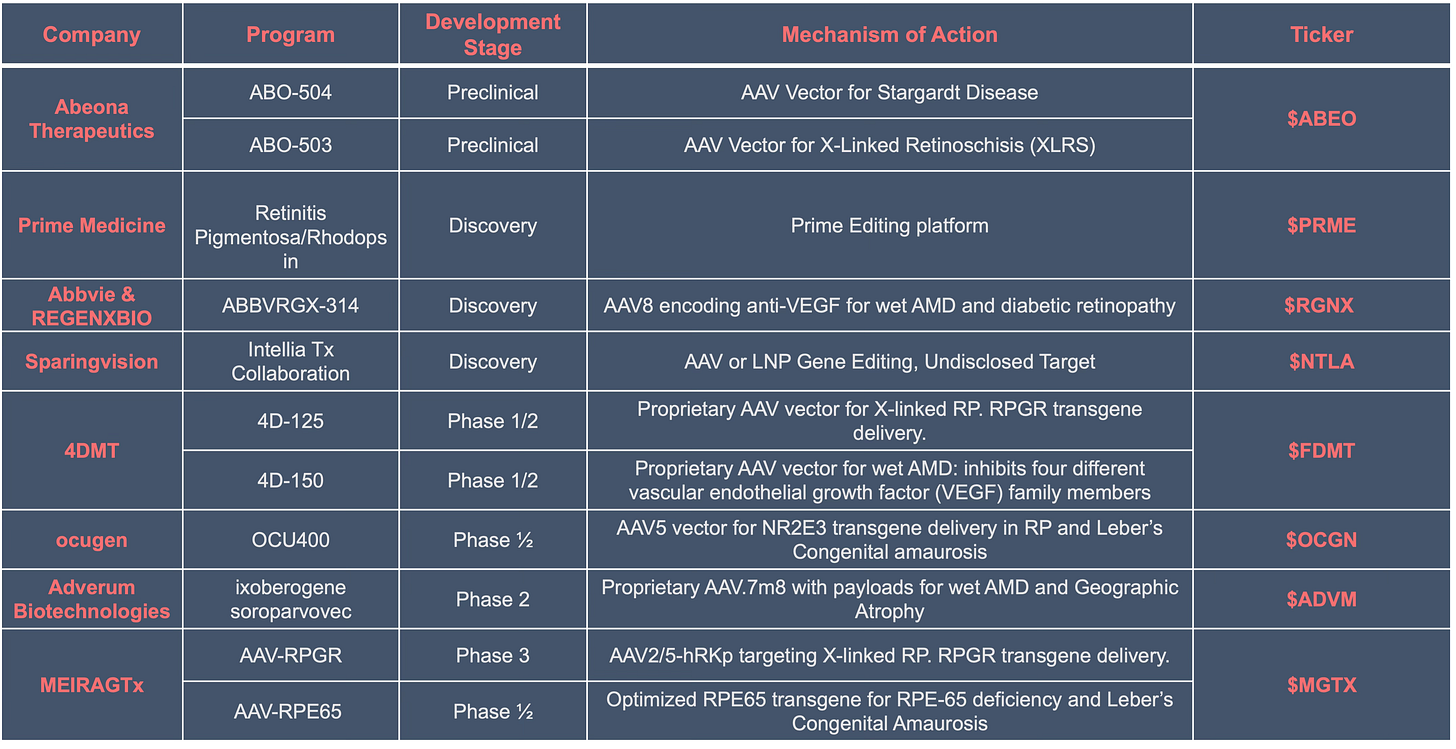
Nonetheless, companies are hard at work developing the next ocular gene therapies and below a few public companies worth keep an eye on: